|
Home Biography Research Few Publications Full List of Publications Graphical Abstract Project Funding Collaborators Courses Group Members Photo Gallery |

|
|
|
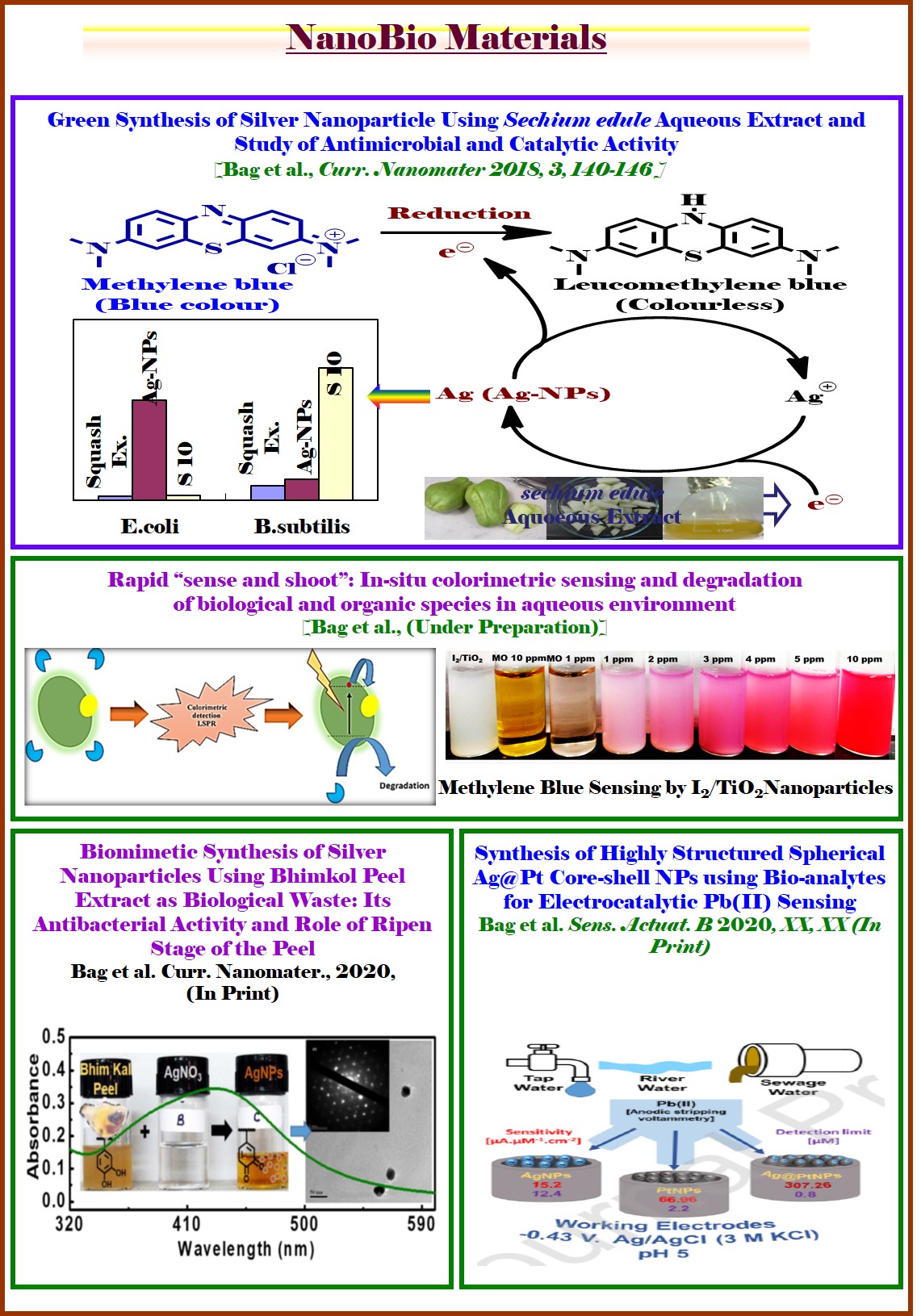
| Nano-Photocatalysis and Nano-Biomaterials |
|
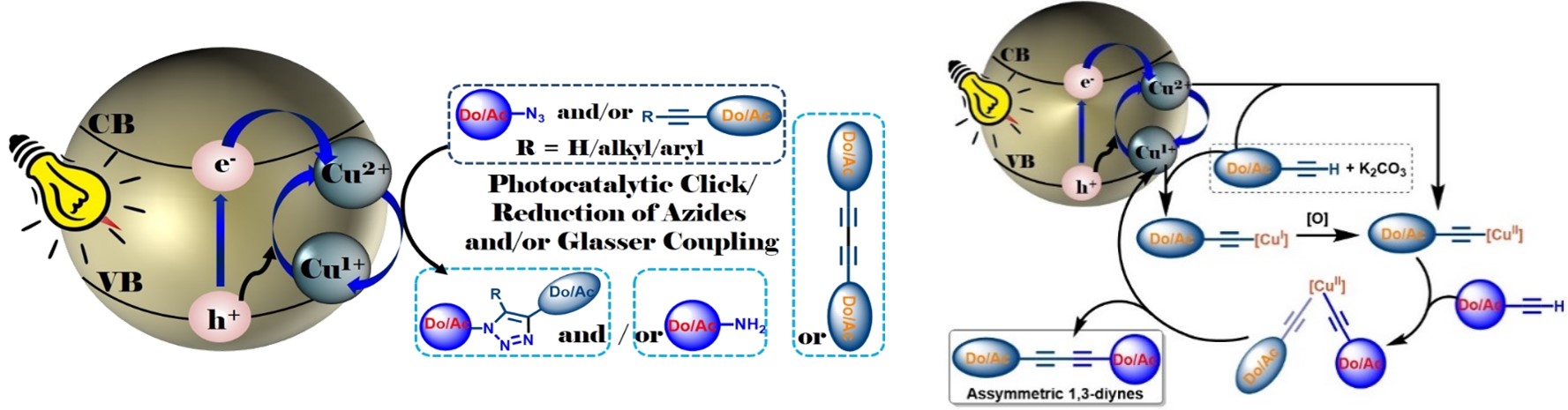
Overview: We have envisioned that application of photocatalyst in organic synthesis is a challenging alternative and green approach. Synthesis of industrially important organic compounds through heterogeneous photo-catalytic route is expected to revolutionize the socio-economic backdrop by harvesting energy and reducing the synthetic process cost. Therefore, presently at my lab I have also started to synthesize efficient nano-photocatalysts and thereby investigate in developing new methodologies, involving visible light assisted heterogeneous photocatalysis to synthesize medicinally, industrially and bio-geochemically important compounds (a) “Photocatalytic Click Reaction and Glaser Coupling Using a Smart and Multitalented Nanocomposite Photocatalyst” (2019, Communicated). (b) Furthermore, the development of cheap and effective biomaterials for burn wound healing applications in defence sector is an attractive research area in recent time. Toward this end, we also have started research on developing effective nano-biomaterials as an alternative therapeutic. We believe that the synthesized biomaterials would help in fluid resuscitation, provide nutritional support and wound care as well as impose control of infectious agents (Curr. Nanomaterials 2018, (10.2174/2405461503666181002115659); J. Electroanal. Chem. 2018, 827, 181; Environmental Progress & Sustainable Energy 2017, 36, 192; Ongoing Project). Photocatalyst: Unifying both the properties of CuI and CuII in a catalytic cycle I recently have developed a unique nano-photocatalytic system for serving twofold jobs in green solvent with high efficiency, selectivity and fast reaction kinetics. Thus, a heterogeneous photocatalyst based on CuII ion doped TiO2 nanocomposite [CuII@TiO2] [CTOCHNC Photocatalyst] has been synthesized which is found to be highly efficient for carrying out base-free copper catalyzed azide-alkyne 1,3-dipolar cycloaddition (CuAAC) reaction in aqueous environment. The same catalyst is also useful for the generation of unsymmetrical 1,3-bisalkynes with excellent selectivity via hetero-Glaser coupling reaction in presence of environmental benign K2CO3 base. Both the reactions feature high product selectivity, mild reaction conditions in green solvent, wide substrate scope, excellent turn over number, high yields, fast reaction kinetics and good functional-group compatibility. Mechanistically, upon light irradiation (λ = 400 nm), the TiO2 nanocore releases electron from its valence band to the conduction band which in turn photochemically reduces CuII to CuI achieving spatial and temporal control over the CuAAC reaction. On the other hand, generated hole and/or oxygen in air as a green oxidant, next, maintains the CuI to CuII catalytic cycle as feed for the Glaser coupling reaction. The working principles are schematically shown in the figure below.
Thus, cooperative effect of both the generated electron and the hole continually maintains the catalytic cycle without needing of O2 from the air achieving spatial and temporal control over the Click as well as hetero-selective Glaser coupling reactions. In presence of hole-quencher such as tBuOH the catalyst need O2 to maintain the catalytic cycle. Thus, this catalyst is advantageous over other reported catalytic systems and can be treated as a complement to existing methods. Especially, it is much more beneficial for the synthesis of unsymmetrical-1,3-diynes. Because, the existing Cadiot–Chodkiewicz protocol for the synthesis of unsymmetrical 1,3-diynes suffers from several shortcomings and thus is impractical due to the low stability of the prefunctionalised alkynes, high cost, tedious work-up process and green demand. With this backdrop, the developed catalyst by us would surely attract enormous academic and industrial interest. As a demonstration, my research group has already synthesized a series of donor-acceptor chromophore decorated triazoles (35 nos.) and unsymmetrical-1,3-diynes (30 nos.) including few triazole/diynyes decorated fluorescent unnatural amino acids and nucleosides. More interestingly, the catalyst is also efficient for internal alkynes for Click reactions. Furthermore, both the processes work well under physiological conditions expanding the scope of applicability in labelling functionalized biomolecules and hence makes the catalyst universally appealing. Patent application for the catalyst, and the process is underway.
|