|
Home Biography Research Few Publications Full List of Publications Graphical Abstract Project Funding Collaborators Courses Group Members Photo Gallery |

|
|
|
| EXPANSION OF THE GENETIC CODES |
|
Overview: The design and encoding of fluorescent unnatural amino acids toward expanding the genetic code would expand proteins functions and biotechnological applications. Our observation on the installation/modulation of emission response via click reaction (J. Org. Chem. 2011, 76, 3348) has led us to design triazolyl donor-acceptor unnatural amino acids with novel solvatochromic fluorescence properties. To this end we have succeeded in achieving the following innovative results with immediate/long term applications (J. Photochem. Photobiol. A: Chem. 2019, 378, 171). The conceptual development of fluorescent triazolyl donor-acceptor chromophore decorated unnatural amino acids is the unique introduction to the expanded genetic code [Ref. 1, 8-9, 33-34, 38]. We have exploited Triazolo amino acids as new and novel constrained molecular scaffolds for β-turn peptidomimetics [Ref. 11, 17, 24, 28, 33, 34, 36]. We have shown that the readily accessible triazolo-β-aza-e-amino acid (AlTAA) and its aromatic analogue (ArTAA) in the peptide backbone mark a novel class of unnatural peptide building blocks with properties of functioning as conformationally constrained molecular scaffold which in peptide backbone induce desirable secondary structures in small linear peptides. This is demonstrated for AlTAA in a Leu-enkephalin analogue with purely β-turn conformation wherein the scaffold replaces the original Gly-Gly dipeptide sequence. Interestingly, the scaffold AlTAA also induces a β-turn structure in a pentapeptide wherein FRET is established between two terminal fluorescent triazolyl unnatural amino acids. Restricted rotation about C(aminophenyl)-C(triazole) bond induces chirality and turn conformation onto the achiral aromatic scaffold, ArTAA, which in a short tripeptide backbone acts as a β-turn mimic as a β-sheet nucleator. Under study are the explorations of these turn mimetics and other analogues in details and the sequence specific DNA binding event of tetra-amides of these molecular scaffolds which might lead to the generation of a new family of distamycin analogues [Chem. Commun. 2015, 51, 5242; Invited in Special Issue: Emerging Investigators Issue.]. The scaffold AlTAA in a Leu-enkephalin analog sequence represents a Gly-Gly dipeptide mimic and induces purely β-turn conformation [Ref. 36]. Interestingly, the scaffold AlTAA also induces a β-turn structure in a pentapeptide wherein Förster Resonance Energy Transfer (FRET) process is established between two terminal fluorescent triazolyl unnatural amino acids [Ref. 11]. On the other hand, the axially chiral aromatic amino acid scaffold (ArTAA) adopts a hairpin turn shape and acts as a β-turn mimic as a β-sheet nucleator when present in a peptide backbone [Ref. 34, 36]. These inventions along with two consecutive communications in a single issue (2014) inspired the then Editor of Chem. Commun. Dr. Robert D. Eagling. Hence he invited me for an article in a Special Issue entitled: “Emerging Investigators Issue” in which he contributed vis-a-vis the introduction of novel scaffolds amino acids [Ref. 36].
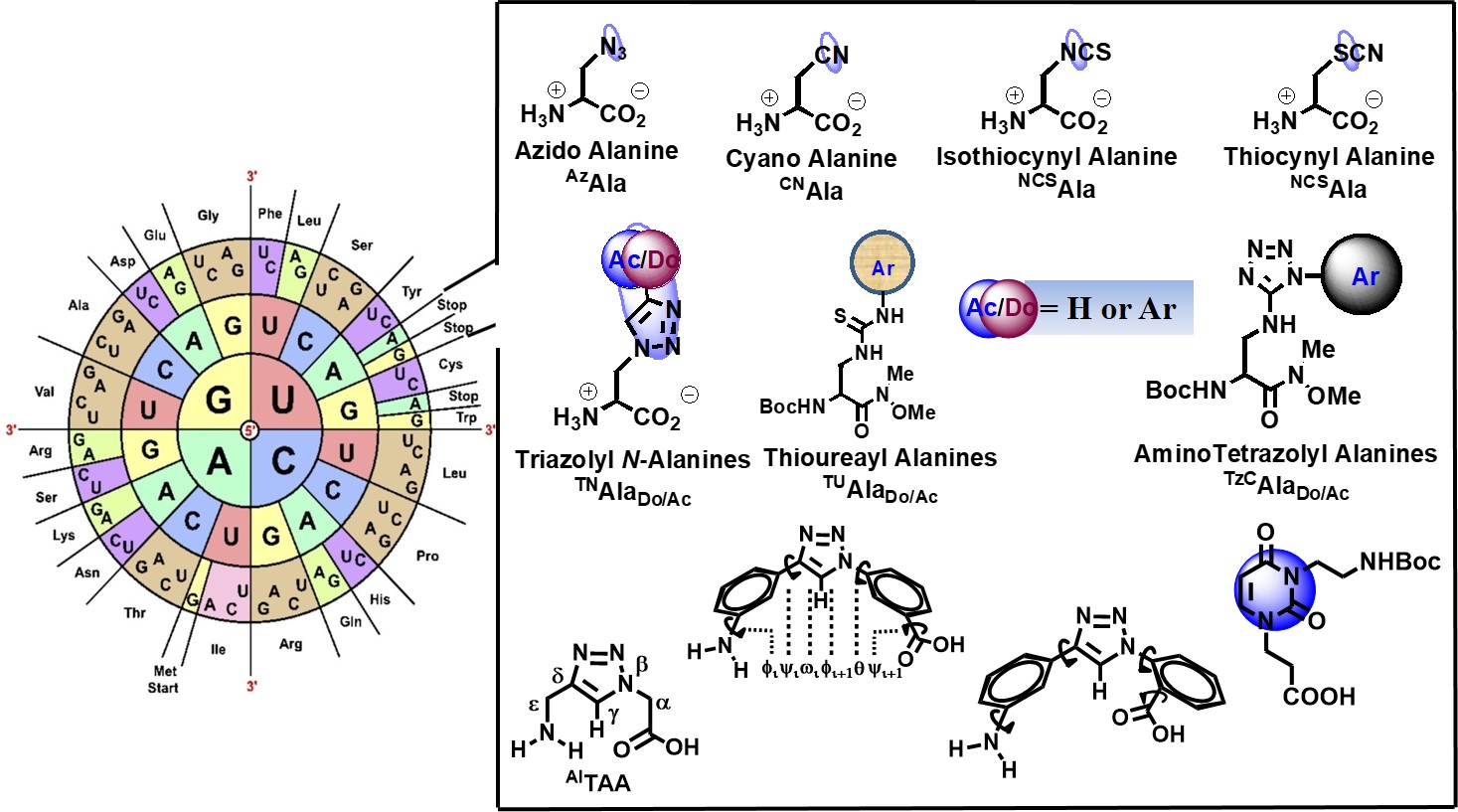
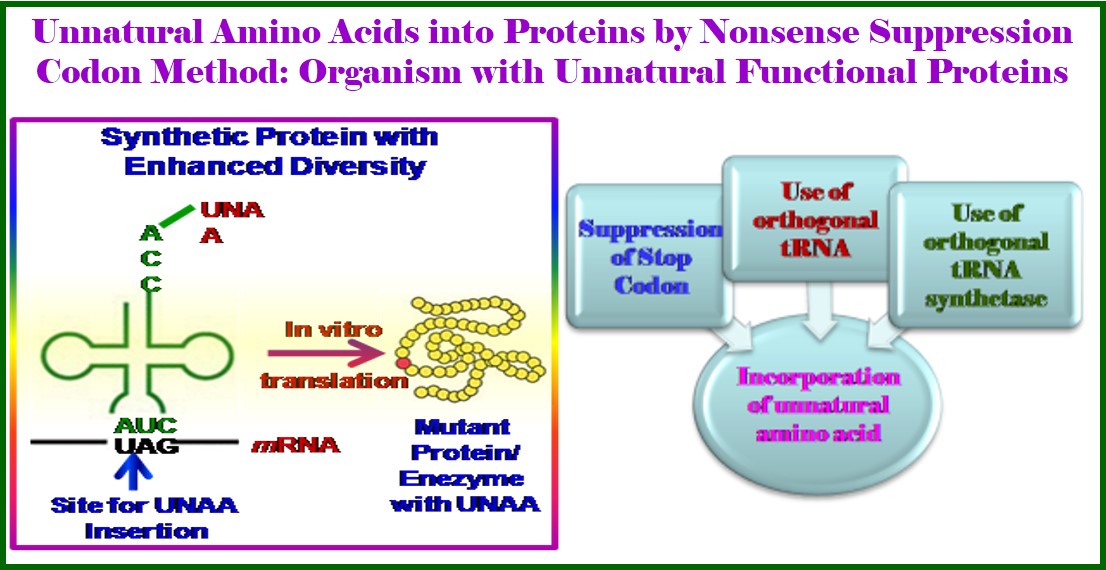
As a part of our research towards expansion of genetic codes, we recently reported the design of unnatural amino acids with small side chain functionalities useable for further synthesis and as spectral probes. Towards this end, recently, we reported the synthesis of an unexplored unnatural amino acid, isothiocyanyl alanine (NCSAla = Ita) as an synthetic intermediate for another class of unnatural amino acids, thioureayl alanine ( TUAla = Tua) which is further exploited to generate third class of unnatural amino acids, amino tetrazolyl alanines (ATzAla = Ata) in very good yield. Thus, a variety of aliphatic and aromatics substituted thioureayl alanines and aromatic substituted amino tetrazolyl alanines have been successfully synthesized in good to excellent yields. The –NCS functionality is also shown to have the solvatochromic IR sensitivity in a peptide which further can be utilized as IR sensitive spectral probe to investigate structure, dynamics, localization, and biomolecular interactions. The conformational analysis of the tripeptide containing NCSAla/hexapeptide with isothiocyanyl Lysine (NCSLys) show that the side chain –NCS influences the conformation either as PPII or as C7 depending on the polarity of the solvents. Finally, the electrophilicity of isothiocyanyl alanine (NCSAla = Ita)/ isothiocyanyl lysine (NCSLys = Itl)/has further been exploited in labeling/ligation of a short tripeptide as a model site specific labeling at physiological condition (J. Org. Chem., 2017, 82, 12276; Bioorg. Med. Chem. Lett. 2018, 28, 1404). Thus, the unnatural amino acids, such as isothiocyanyl alanine (NCSAla = Ita), isothiocyanyl Lysine (NCSLys), thioureayl alanine (TUAla = Tua), amino tetrazolyl alanines (ATzAla = Ata) are unique addition in the list of small non perturbing unnatural amino acids [Ref. 18, 25]. My group was the first to show that the–NCS functionality of NCSAla or NCSLys can be exploited as an IR sensitive spectral probe to investigate the protein structure, dynamics, localization, and biomolecular interactions [Ref. 13]. Moreover, the electrophilicity of NCSAla and NCSLys has also been exploited for labeling/ligation of short hexapeptide and tripeptide as the model for site-specific labeling at physiological conditions. Our ongoing research focuses on the synthesis of Tetrazolyl unnatural amino acids and other having electrophilic center and penicilanic acid/tetrazolo amino acids as constrained molecular scaffold for β-turn peptidomimetics. The recently developed uracil-amino acid scaffold as a hybrid ensemble acts as β-turn peptidomimetics inducing β-sheet structure in a peptide (Bioorg. Med. Chem. Lett. 2017, 27, 5387). The concept of umbrella covering of electrophilic amide carbonyl between Phe-Pro which is mainly the reactive site for HIV-I Protease can expectedly lead to the generation of new class “steric inhibitors” of HIV-I Protease. The same aromatic amino acid scaffold has been utilized in sensing ethanol in presence of methanol (New J. Chem., 2017, 41, 13391). The ortho-meta analogue also has been shown to induce β-sheet structure and in a design trichromophoric pentapeptide it showed relay FRET emission (RSC Advances 2016, 6, 72654). It interesting to note that our unnatural nucleobases could serve as a part of codons in mRNA, which can be exploited to code for his novel triazolyl unnatural amino acids/ NCSLys using an engineered orthogonal aminoacyl-tRNA synthetase (AARS) and tRNA pair. Such a study would ultimately allow them to translate the genetic alphabet into genetic code leading to the generation of semisynthetic organisms. I am delighted to know that he is recently engaged in designing peptidomimetic inhibitor drugs (PIDs) for COVID-19 as a part of his contribution to society.
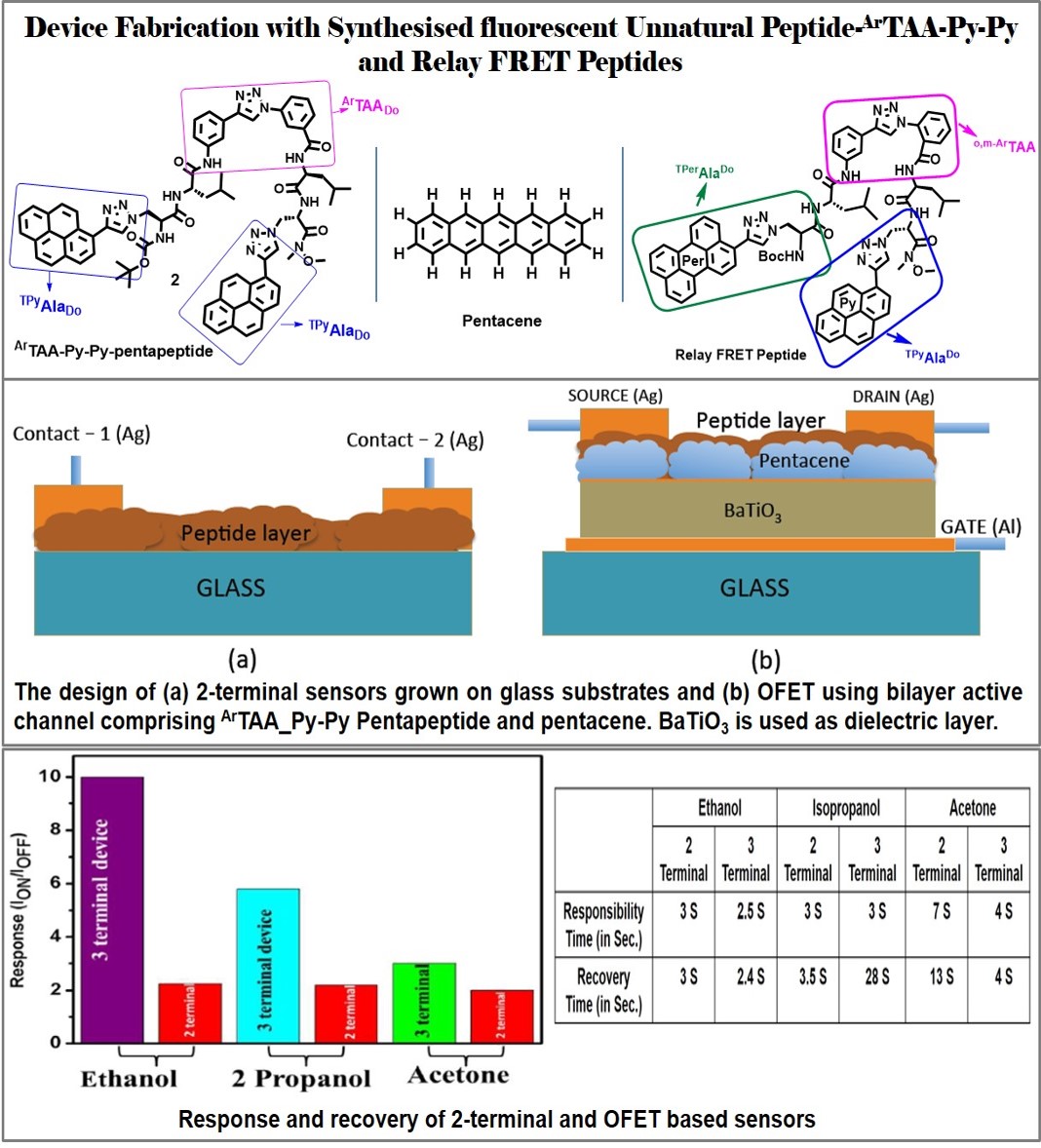
Application- OFETs Sensor for Biomedical/Defense Application: The axially chiral amino acid scaffolds ArTAA has been elegantly exploited for discrimination of methanol from ethanol both in liquid and gaseous state via a switch on fluorescence response [Ref. 24]. This is a simple technique that would be attractive to develop a system that could estimate ethanol content on-the-spot. This work has recently been acknowledged as “worthy of commendation” by Dr. Wael A., Chief Author, London Journals Press. He also mentioned, “I found your research work to be distinctive and can indeed be significant for fellow researchers and scientists working in the same domain”. Thus, the Editorial Board has recognised Dr. Bag under "Quarterly Franklin Membership" (Membership ID#BP95271). These chiral amino acids and fluorescent peptides are in use to develop practical sensory devices based on organic Filed Effect Transistors (OFETs). They have developed a semiconducting bilayer geometry in OFET design. It includes one highly conducting pentacene layer coated with a fluorescent peptide layer (fluorescent ArTAA_Py-Py pentapeptide). Such a device has been tested for the detection of polar gas molecules like alcohol and acetone at room temperature. It also can detect blood proteins in solutions successfully. This is the first example of a peptide based OFET device which is thus unique in design. It is also advantageous in comparison to other available gas sensing devices as it works efficiently at room temperature, exhibits higher selectivity and enhanced sensitivity. The part of the work entitled, “Fabrication of Peptide-based room temperature gas sensors using organic field-effect transistors,” was presented in a conference, “The Swiss Conference on Printed Electronics and Functional Materials (Swiss ePrint 2019), 5th Edition” in Switzerland. The paper has well been appreciated and recognized to offer a Best Poster Award. [Ref. 24, 84].
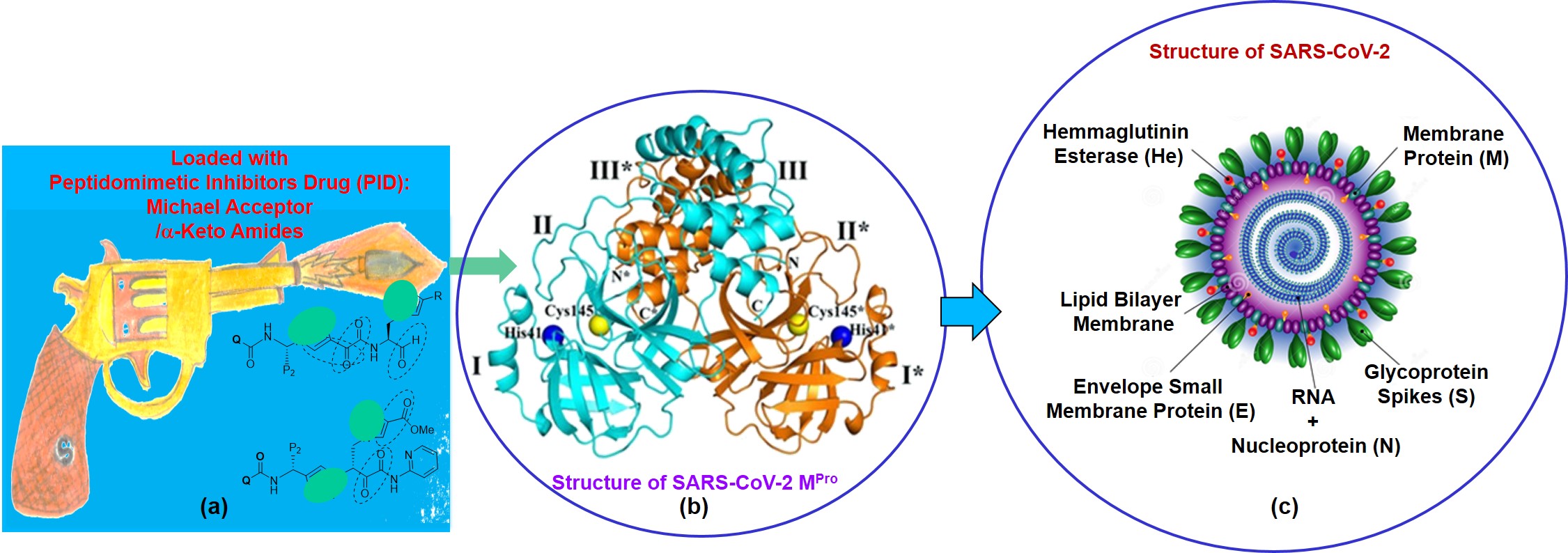
Additionally, we are also working to integrate microfluidic channels to convert OFETs for the rapid detection of biomolecules in the solution phase and also would be extended for the detection of chemical/biological warfare agents. Both the findings are under preparation for filing a patent. Application-Peptidomimetic Drug Design: As a part of amino acid/peptide-based drug development research, I have elegantly exploited some of the developed unnatural aminoacids and designed peptides for studying their biological activity and using as fluorescent probe for peptide-protein/peptide-DNA interaction. Thus, I found and reported that the unnatural triazolyl amino acids and peptides are potent anti-malaria agents against PF14_0660 as a target [Ref. 9]. The developed β-turn peptidomimetic candidates are found to act as β-sheet breaker peptides and could find potential therapeutic applications for the treatment of Alzheimer's disease. My developed β-turn peptidomimetics and Lopinavir analog peptides are found to show potent inhibitory activity against HIV-Protease-I. To contribute to the society during the present world crisis due to COVID-19, I have started the Molecular Docking study of novel SARS-CoV Mpros protease with few of the developed peptidomimetic candidates as a part of drug repurposing program. The results are identified to have significant inhibitory activities against the novel COVID-19 protease. Among all these peptidomimetics tested, the axially chiral amino acid scaffold (ArTAA) and corresponding peptides, Leu-enkephalin analog peptides, have shown strong bonding interaction and high affinity with protease. Thus, these peptidomimetics may be considered as effective inhibitors of COVID-19 protease. Moreover, my group has recently started synthesizing potent a-keto amide analog peptides exploiting their β-turn nucleating scaffold amino acid [Ref. 11, 17, 24, 28, 33- 34, 36, 28] for inhibiting SARS-CoV Mpros protease. This work has recently been submitted to CSIR for a grant. Part of the work is also under consideration in a recently submitted collaborative research project under IRPHA on COVID-19 special call. The research outcome expectedly would have a significant impact on the overall Human Health. We have recently submitted a proposal to CSIR for funding based on "Design, Synthesis and Therapeutic Application of Novel Peptidomimetic Inhibitor Drugs (PIDs) for COVID-19". Our design of peptidomimetic drug inhibitor (PID) against SARS-CoV-2 Mpro main protease is proposed to starts from a knowledge of substrate binding pocket, substrate and mechanism of action of the potent inhibitors N3 and a-ketoamide based peptidic inhibitor. This understanding is a starting point for the development of new SARS-CoV-2 Mpro inhibitors. We propose that the variation of P1/P2 in our design would lead to potent N3-analog and a-keto amide inhibitor, respectively. Initially we want to choose our unnatural triazolyl aliphatic amino acid scaffold (AlTAA) to construct our inhibitor. It is a β-Ala-Gly dipeptide mimic and when present in the backbone of a Leu-Enkephalin analog sequence, it induces β-turn structure, thus acting as β-turn-nucleator.
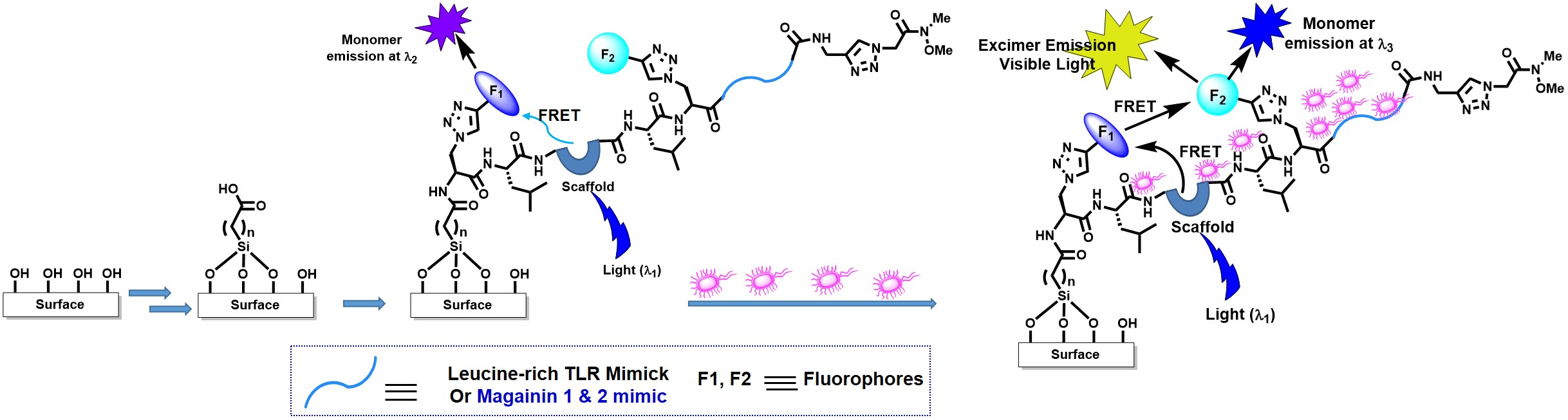
As the SAR-CoV-2 Mpro specifically cleave the peptide bond following a P1-glutamine residue we decided to use a 5-membered triazolyl ring which can be a mimic of amide side chain of glutamine (which can be called as Gln-Tz). This triazole moiety can enhance the power of the inhibitors possibly due to rigid triazole ring which would lead to a reduction of the loss of entropy upon binding to the target protease. Application-Peptide Scaffolds in Photochemistry and Photobiology: Similar to the nucleic acid chemistry, I have also exploited the developed novel fluorescence peptidomimetic candidates for contributing in the field of photochemistry and photobiology. Thus, I have utilized their unnatural amino acid, TCNBAlaAc and TPhenAlaDo in two termini of a tripeptide and elegantly shown the FRET event [Ref. 38]. The tripeptide framework adopted β-turn conformation. The tripeptide containing fluorescent solvatochromic Do/Ac amino acids adopted β-turn conformation wherein we observed FRET process. To the best of our knowledge the conceptual fluorescent unnatural peptide showing FRET process in the β-turn conformation is new. In summary, Förster resonance energy transfer (FRET) was established in the conceptually designed novel unnatural β-turn peptide containing a new class of fluorescent unnatural donor/acceptor amino acids. The FRET process occurred from unnatural amino acid, TCNBAlaAc (FRET donor) to unnatural amino acid, TPhenAlaDo (FRET acceptor). Our developed FRET pair, TCNBAlaAc - TPhenAlaDo, wherein both the partners are unnatural amino acid, is new to the best of our knowledge. As FRET can provide information about peptide/protein conformation, our unnatural amino acid pair (FRET pair) and the FRET peptide might find application in studying solution conformational distribution of an unstructured peptide and FRET based bioassay (Chem. Commun. 2014, 50, 433; Bioorg. Med. Chem. 2016, 24, 3579). We recently published, Relay FRET Event in a Designed Trichromophoric Pentapeptide Containing o-, m-Aromatic-Amino Acid Scaffold which showed first example of relay FRET process in peptide (Chem. Commun. 2018, 54, 9765-9768). Few of our synthesized unnatural triazolyl amino acids found to be active as anti-malaria agent against PF14_0660 as a target (Letters in Drug Design & Discovery 2018, (10.2174/1570180815666180727121200). The same aromatic amino acid scaffold has been utilized in sensing ethanol in presence of methanol (New J. Chem., 2017, 41, 13391). The ortho-meta analogue also has been shown to induce β-sheet structure and in a design trichromophoric pentapeptide it showed relay FRET emission (RSC Advances 2016, 6, 72654). We reported a Dual Door Entry system to excimer emission in a designed triazolo amino acid scaffolded (ArTAA) trichromophoric b-sheet pentapeptide. In our design, two fluorescent unnatural amino acids (TPyAlaDo) are embedded into two arms of a novel chromophoric scaffold, ArTAA, via an intervening natural amino acid, leucine. The unnatural fluorescent pentapeptide shows an excimer emission either via Forster resonance energy transfer (FRET) from the scaffold (ArTAA) acting as donor or via direct excitation of an acceptor chromophore, TPyAlaDo. Moreover, it serves as an effective fluorescence light-up probe for studying protein-peptide interaction (RSC Advances 2016, 6, 72654). The designed fluorescent pentapeptide to show an excimer emission either via FRET from the scaffold (ArTAA) acting as donor or via direct excitation of an acceptor chromophore, TPyAlaDo is his novel addition in the field. This excimer emission is the generalization of the applicability of the Dual Door Entry System, which has initially been invented for exciplex emission in a DNA based design [Ref. 34, 39]. Moreover, it serves as an effective fluorescence light-up probe for studying protein-peptide interaction. The beauty of my peptide probe lies in the fact that it maintains the conformation and photophysical property while interacting with protein biomolecule. Such a unique probe would provide fundamental guidelines for the design of more conceptual fluorescent peptide-probe that would hold great promise for application in chemical biology [Ref. 11, 17, 34, 36, 38-39]. We recently established and reported the concept of relay FRET event in a designed trichromophoric pentapeptide containing o-,m-aromatic amino acid scaffold in the backbone as a novel a β-turn mimetic β-sheet folding nucleator. This system would find applications in studying fundamental processes involving interbiomolecular interactions in chemical biology (Chem Commun. 2018, 54, 9765). We also showed distance dependent FRET efficiencies in donor/acceptor pairs in the two termini of designed fluorescent pentapeptides and found that the flexible N-Terminus of the scaffold in a pentapeptide has led to higher FRET efficiency and makes it different from other peptide with flexible C-terminus (J. Photochem.Photobiol.A. Chem. 2019, 378, 171). The conceptual fluorescent unnatural amino acids (UAA) and unique triazolyl-/uracil amino acid as β-sheet/β-turn mimetic scaffolds and peptides might be the solution of extrinsic fluorescent labels for application in real-time imaging of live cell, generating the fluorescent protein biosensor, high-throughput screening, diagnostics, proteomics, and for monitoring and understanding biological events accompanying interbiomolecular interactions. Fluorescent Antimicrobial Peptide-based Bacteria/Virus Sensor: We are now engaged in developing bioinspired materials and antimicrobial peptide based biomaterial for possible clinical use. We are engaged in creating new and novel therapeutic alternatives against the recent rise of multidrug-resistant microbial strains. We have put up a project proposal based on the recent call for Team Science Grant & Clinical/Public Health Research Centres Grants from IndiaAlliance DBT. We want to exploit the β-turn/β-sheet Peptidomimetics developed by Dr. Bag, Peptoids by Prof. Aaron and the Surface tethering techniques by Prof. Lalit at BSBE Department of IITG to create therapeutic surfaces. The bioinspired materials based on fluorescent peptidomimetics developed by me could also find applications in sensing and medical diagnostic purposes. This project would have great impact to resist virus protein capsids, such as SARS-CoV-2 and similar SARS viruses, from surface attachment.
|