|
Home Biography Research Few Publications Full List of Publications Graphical Abstract Project Funding Collaborators Courses Group Members Photo Gallery |

|
|
|
| CHEMISTRY OF ENEDIYNE ANTICANCER ANTIBIOTICS |
|
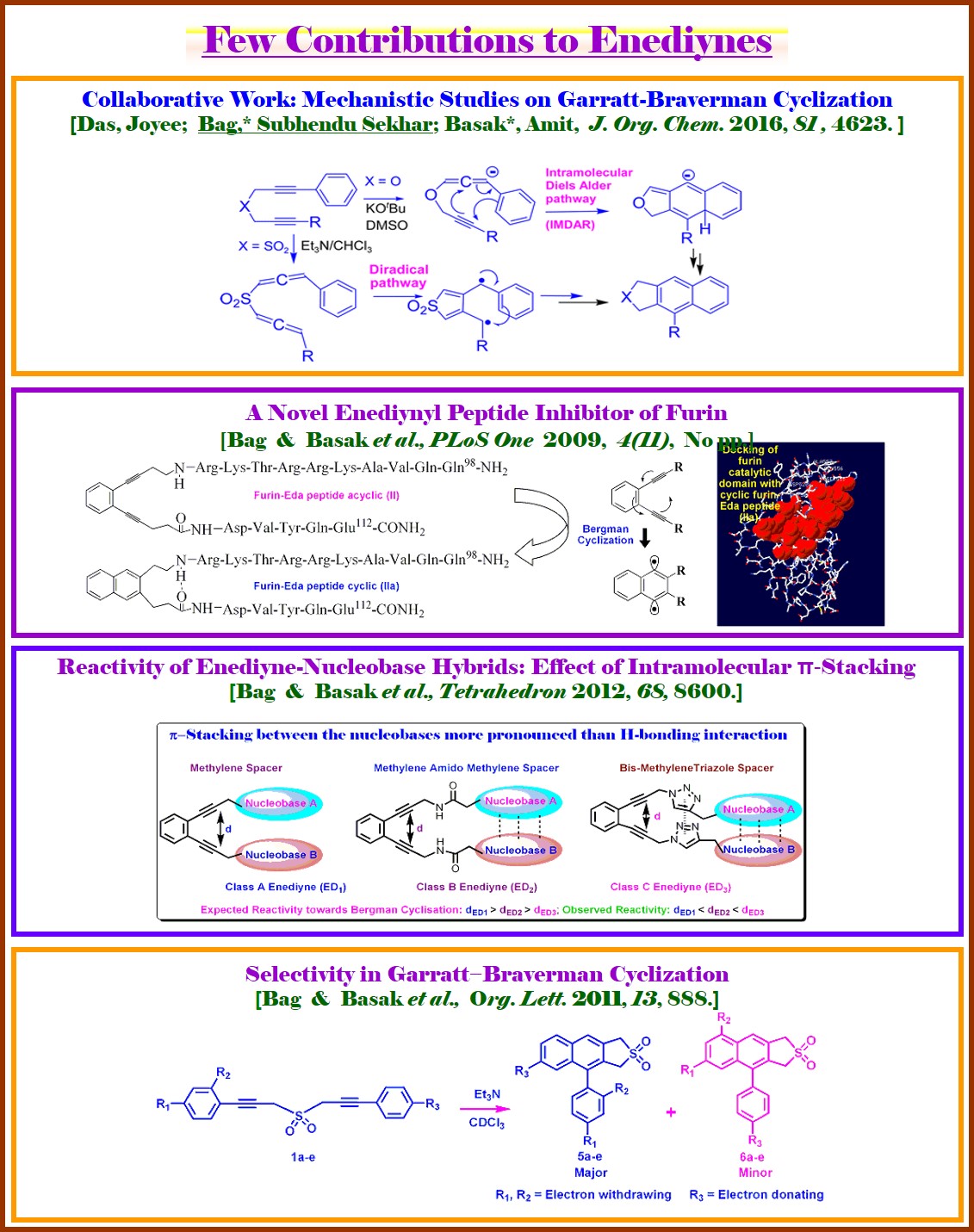
Overview: We are also engaged in designing the Enediyne-anticancer-antibiotics and study their reactivity toward the generation of biradical which is the source of cleaving the Cancer Cell DNA, thus showing the anticancer activity. The importance of enediyne research in respect of cancer treatment is clear from the approval of Mylotarg [A Calichiamicine (containing the Enediyne warhead)-antibody hybrid] by US FDA in 2000 as a potent drug in treatment of acute myelogenous leukemia from 2000-2010 (drug has been withdrawn from the market in June-2010 because of its other side effects). Thus, we have synthesised bispropargyl sulfones equipped with aromatic rings of dissimilar nature. Under basic conditions, these sulfones isomerized to the bisallenic sulfones, creating a competitive scenario between two alternate Garratt−Braverman (GB) cyclization pathways. The observed product distribution ruled out the involvement of any ionic intermediate and supported the diradical mechanism with greater involvement of the electron-rich aromatic ring via the more nucleophilic radical. DFT-based calculations supported the diradical mechanism along with the observed selectivity (Org. Lett. 2011, 13, 888). We also were able to develop enediynyl peptide inhibitor of furin that blocks processing of proPDGF-A, B and proVEGF-C (PLoS One 2009, 4(11), No pp.). Another work highlighting the effect of p-stacking between the appended units and same between the Click-Chemistry derived linkers in accelerating the reactivity of the designed enediynes, is published (Tetrahedron 2012, 68, 8600). Thus, we, for the first time, have fundamentally shown that the reactivity of enediyne–nucleobase hybrids is greatly influenced by the stacking interaction among the nucleobase pair. In relation to study the mechanism of GB cyclisation we reported the results of extensive multi prong studies involving fate of deuterium labeled substrates, EPR, trapping experiments and LA-LDI mass spectrometry to sort out the controversies relating to the mechanism of Garratt-Braverman cyclization in two systems, namely bis-propargyl sulfones and ethers. The results are in conformity with a diradical mechamism for the sulfone, while for the ether, the anionic [4+2] appears to be the preferred pathway. This shows that the mechanistic pathway towards GB cyclization is dependent upon the nature of hetero atom (O or S in sulfone) bridging the propargyl arms (J. Org. Chem. 2016, 81, 4623). The reactivity of indole based bis-propargyl ethers 4a-4g under Garratt-Braverman condition (KOBut in refluxing toluene) has been studied. Interestingly, these propargyl systems with one arm attached with substituted 3-indolyl derivatives leaving the other arm unsubstituted produced the 3,4-furan fused dihydrocarbazole derivatives 6a-6g (and not the expected carbazole derivatives) as the predominant product (70–82%) making this methodology to access such derivatives an attractive route. The results are supported by computational studies and some of the carbazole derivatives showed good antifungal activities (Tetrahedron, 2018, 74, 3543-3556). My unique NPTEL web based course on “Bio-Organic Chemistry of Natural Enediyne Anticancer Antibiotics” has attracted immediate academic interest [http://nptel.ac.in/courses/104/103/104103068/]. Our contribution to radical generating hybrid organics such as enediyne-nucleoside/enediyne-amino acid hybrid molecules would find potential application as anticancer agent.
|