|
Home Biography Research Few Publications Full List of Publications Graphical Abstract Project Funding Collaborators Courses Group Members Photo Gallery |

|
|
|
| CHEMISTRY OF β-LACTAM ANTIBIOTICS |
|
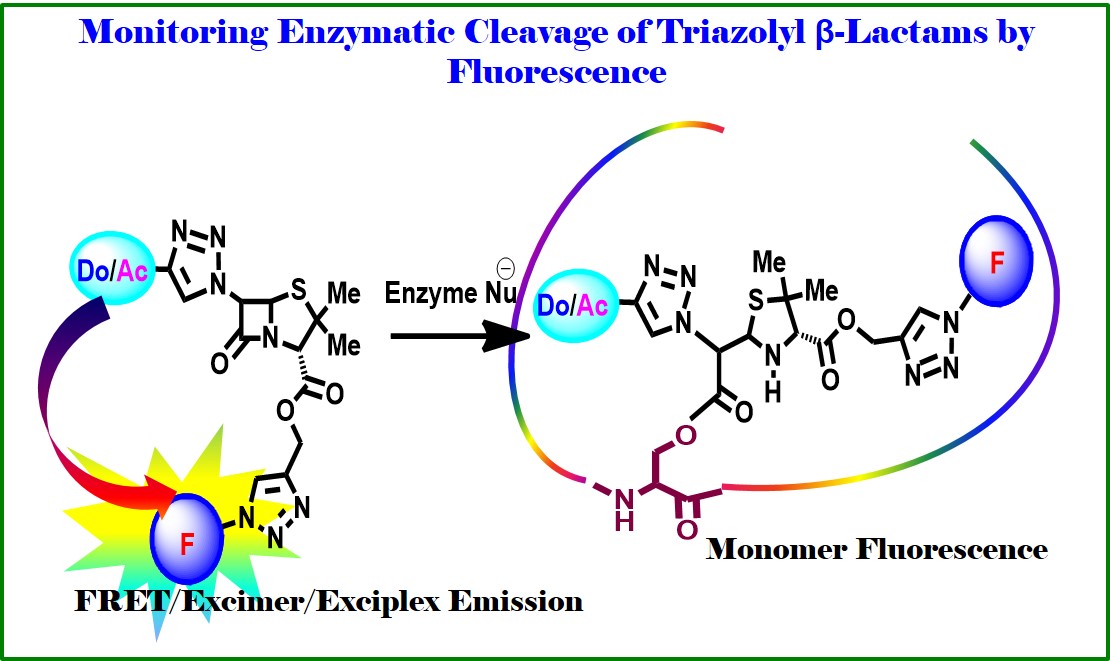
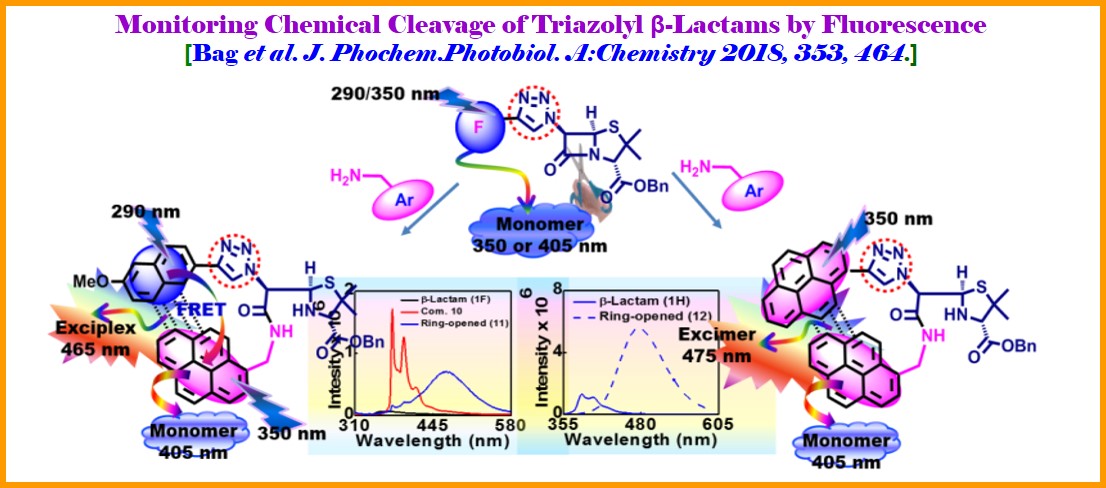
Overview: Besides the nucleicleoside/peptidomimetic based drug design and development program, we are also involved in designing β-lactam antibiotics such as to develop potent fluorescent b-lactam antibiotics and study their chemical, photophysical, and biological activity. Thus, we have shown that the antiaromaticity is playing the prime role in suppressing the b-effect of silicon in 3-silyl monocyclic b-lactam system. A theoretical calculation on antiaromaticity also supported our experimental kinetics of nucleophilic and radical substitution reaction at 4-position of the 3-silyl monocyclic b-lactam system. Our study shed some light to support the well believed radical path of enzymatic reaction for the synthesis of Clavulanic Acid, the 4th generation antibiotic (Org. Lett. 2009, 11, 5722). We recently reported the design, synthesis of C6-triazolyl donor-acceptor fluorescent penicilines. Two of the fluorescent penicilines have been utilized for studying the photophysical outcome after ring cleavage by an external fluoregenic amine as nucleophile. Thus, the chemical cleavage of β-lactam rings is signaled by interesting photophysical phenomena-dual path to exciplex emission or via excimer emission (J. Phochem.Photobiol. A: Chemistry 2018, 353, 464). Overall, our approach would represent a broad diagnostic platform that might form the basis for the development of other similar assays for a variety of targets and antibiotics.
Other related works “Use of Azido Naphthalimide Carboxylic Acids as Fluorescent Template with Built-in Photo-reactive Group and Flexible Linker Simplifies Protein Labeling Study: Applications in Selective Tagging of HCAII and Penicillin Binding Proteins” published in Chemical Communications 2017, 53, 13015 (Outside Back Cover). This work describes the synthesis of azidonaphthalimide carboxylic acids acting as fluorescent template with built-in photo-reactive group and linker thus simplifying the design of protein labeling agents. These were separately connected to selectivity hands like a sulfonamide and ampicillin for successful labeling/detection of HCAII and PBPs respectively. It is a noteworthy contribution in the field of proteomics for understanding cellular processes and also in drug design. This study makes the template universally appealing and shed light for the development of selective probes for capturing β-lactamase Now a day there is no rapid and effective test evaluating antibiotic susceptibility. As a result, doctor has to prescribe antibiotics empirically. This might lead to treatment failures due to increasing antibiotic resistance, and evolution of drug-resistant bacteria. Therefore, the development of a rapid method to detect β-lactamase and to assess activity of β-lactam antibiotics is highly necessary which could provide an approach for targeted prescription of antibiotics. Toward this end, we are in a process of development of method for monitoring of β-lactamase activity by fluorescence technique. Our methodology focuses on the FRET/Excimer emission. The study expectedly would establish a rapid test for β-lactamase detection and prediction of antibiotic activity. Overall, our approach would represent a broad diagnostic platform which might form the basis for the development of other similar assays for a variety of target, pathogens and antibiotics (J. Photochem. Photobiol. A 2018, 353, 464.)
.
|