
Bio Process Analytical Technology (BIOPAT) Laboratory
Phone: +91-361-2582226 (O)
Email : senthilkumar[AT]iitg.ac.in
Research Area
D-Lactic acid production
Biotherapeutics
Human Interferon α2b
Human interferon alpha 2b (huIFNα2b) is an intronless sequence that encodes a polypeptide of 165 amino acids. It can trigger multiple responses that could be antiviral, antibacterial, antiproliferative, or even immunomodulatory. Therefore, huIFNα2b either on its own or in combination with other interferons is being extensively used in prophylaxis of type B and C hepatitis, several cancers such as melanoma, AIDS-related Kaposi sarcoma, chronic myeloid lymphoma, and angioblastoma. Our group successfully engineered a recombinant glycosylated huIFNα2b producing strain. High cell density cultivation of glycoengineered Pichia pastoris embraced by PAT framework produced a significant improvement in huIFNα2b titer (1.4 g/L) by application of robust control strategy.
Currently, our research is directed towards developing control strategies to maintain the tight control over specific growth rate to ensure the consistent product quality.
Cloning, expression, purification and characterization of recombinant human interferon alpha 2b in Pichia pastoris
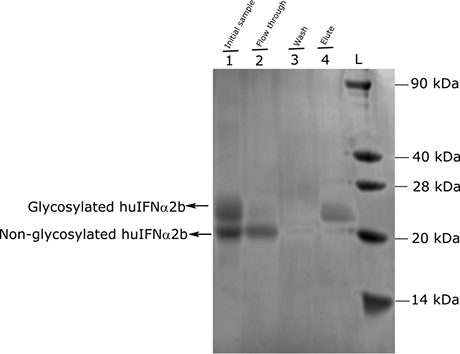
SDS-PAGE of fractions collected during Concanavalin A purification of glycosylated huIFNα2b (SuperMan5) from the pooled fraction of His-tag affinity purification elutes
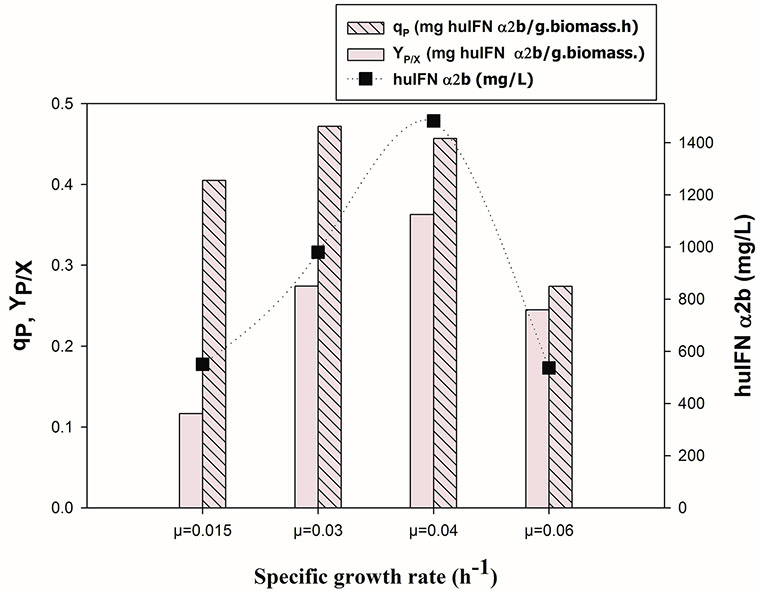
Influence of different specific growth rate (μsp) on huIFNα2b titer, specific productivity (qP) and protein yield coefficient (YP/X).
Heparosan
Heparosan is an anionic, un-sulfated precursor of heparin. It is composed of repeating disaccharide units of D-glucuronic acid and N-acetylglucosamine. Traditionally, heparin was derived from animals but caused serious adverse reactions leading to hypotension and the death of the patients. Chemoenzymatic synthesis of heparin emerged as an attractive alternative that requires heparosan as a starting molecule. Although significant yield was achieved in E. coli K5, it suffered severe disadvantages of causation of urinary infection in humans, secretion of exo and endotoxins which hindered its use. The global heparosan market is expected to increase because of its tremendous demand in the biopharmaceutical industry. Using metabolic engineering approaches, we successfully expressed heparosan in Bacillus megaterium. Currently, novel bioprocess strategies are aimed in order to enhance its production.
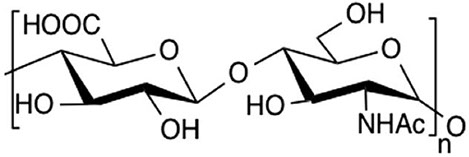
Molecular structure of heparosan composed of repeating disaccharide units of GlcA and GlcNAc linked by β (1→4) and α (1→4) glycosidic bonds
Lactic acid bacteria (LAB) a viable option for heparosan production. It has grabbed much attention because of their reputation for being “GRAS”. GRAS organisms are commonly preferred to produce food grade, pharmaceutical grade products owing to its safety and availability of wide range of genetic manipulation tools. Presently, we are targeting LAB for heparosan production using metabolic engineering approach.
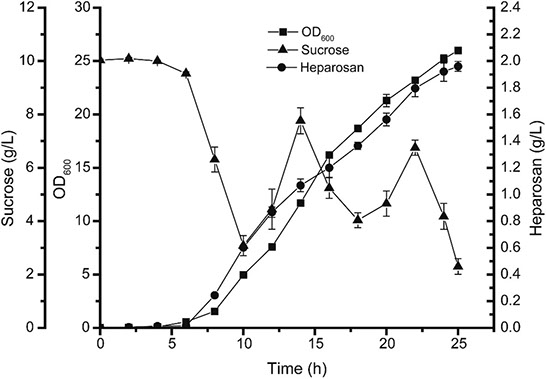
Heparosan production by recombinant B. megaterium in a lab scale bioreactor
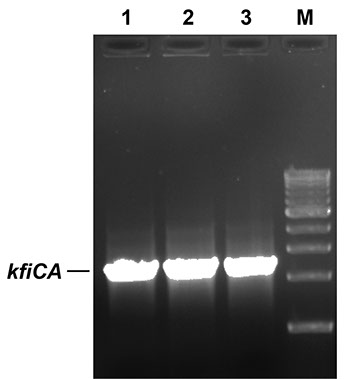
PCR confirmation of E. coli kfiC and kfiA genes in B. megaterium
Copyright @ BioPAT LAB, IIT Guwahati.