Applications of porous metal-organic frameworks (MOFs) in (A) fluorescence sensing, (B) adsorption and (C) catalysis:
Metal-organic frameworks (MOFs), which are a relatively new class of highly crystalline and extraordinarily porous (specific surface area up to 5000 m2 g-1) materials, have
attracted tremendous research interest in the last two decades among an enormous number of international research groups owing to their potential applications in a wide
range of areas such as gas storage, separation, catalysis, ion-exchange, sensing, polymerization and drug delivery. Their three-dimensional framework structures (Figure 1)
are constructed from metal ions or metal clusters interconnected by rigid polytopic organic ligands. Due to the availability of a huge combination of metal ions and organic
linkers, a broad variety of porous MOF structures having different type of pore systems have been reported to date. In sharp contrast to conventional porous adsorbents
such as zeolites (specific surface area < 1000 m2 g-1), mesoporous silicas, carbon nanotubes and activated carbons (specific surface area up to 3500 m2 g-1), the pore sizes
(hence, porosity and specific surface area) and pore surface properties of MOFs can be tuned by varying the sizes of the organic ligands and by attaching different
functional groups (having different sizes, polarities, hydrophobicities/hydrophilicities, acidities, etc.) to the organic linker, respectively. Endowed with the extraordinary
chemical and structural tunability, MOFs might become future "super-adsorbents", "super-catalysts" or "super-sensors".
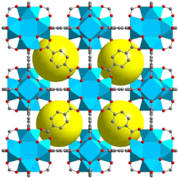
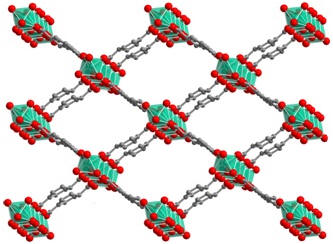
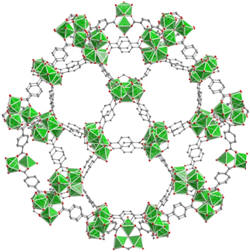
Figure 1. Structures of representative highly stable and porous MOFs (left to right: Zr-UiO-66, Al-MIL-53 and Cr-MIL-101).
(A) Applications of metal-organic frameworks in fluorescence sensing:
The porous crystalline structures of metal-organic frameworks (MOFs) provide several advantages over other sensor materials. The ability to tune the sorption properties of
MOFs offers a high degree of molecular specificity and selectivity for the detection of analytes. In addition to size-selectivity of MOFs, their large surface areas along with
confinement of the analytes inside the cavities can potentially result in highly sensitive detection of analytes. Moreover, the immobilization of functional sites such as Lewis acidic
or basic and open metal sites inside porous MOFs can effectively enable specific detection of analytes. Furthermore, the incorporation of highly conjugated organic ligands in the
rigid frameworks of MOFs causes stronger emissions. Above all, the almost infinite combinations of organic ligands and metal centres render tuning of valance and/or conduction
band and hence the optical band gap, which is very important for sensing applications.
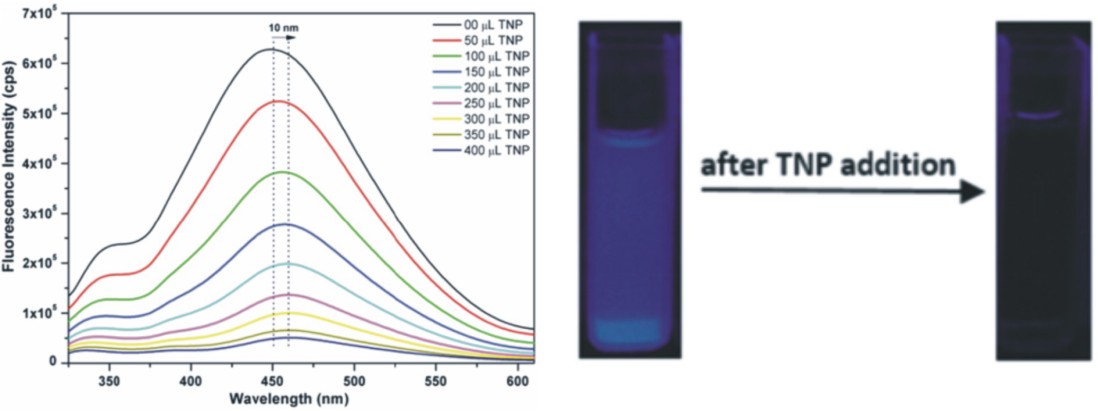
Figure 2. (left) Fluorescence quenching of a MOF upon the addition of 2,4,6-trinitrophenol (TNP). (right) Digital photographs of fluorescence curettes under UV light before and after
addition of TNP.
(B) Applications of metal-organic frameworks in gas/vapor/liquid adsorption:
Owing to their exceptionally high specific surface areas and micropore volumes, MOFs are promising candidates for industrial adsorption/separation of gases (N2, CO2, CH4,
CO, H2, NO, C2H2, NH3, H2S, hydrocarbons, etc.), vapors and liquids (e.g., water and organic solvents). The separation in both rigid and flexible MOFs are governed by
several factors such as size/shape exclusion, adsorbate-framework interactions and adsorbate-specific gate opening pressures. Based on these parameters, MOFs have been
able to perform several commercially relevant gas (CO2/CH4, H2/N2, N2/O2, H2/CO, etc.) and liquid (separation of xylene isomers, separation of alcohols from water, etc.)
separation processes. Recently, MOF materials have been employed as fillers for membrane-based gas separation processes, which are advantageous for large-scale
industrial applications. Furthermore, MOFs have shown great potentials for the adsortive removal of common hazardous materials such as NOx, SOx, COx, H2S, volatile
organic compounds (VOCs), nitrogen-containing compounds (NCCs), sulfur-containing compounds (SCCs), dyes, pharmaceuticals and personal care products (PPCPs), etc. In
Figure 2, a typical N2 adsorption isotherm for a representative MOF (here UiO-66) and its separation power for the CO2/CH4 gas mixture (as suggested from single-
component gas adsorption isotherms) are demonstrated.
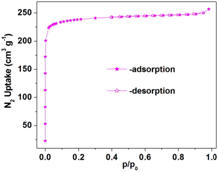
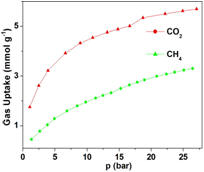
Figure 2. (left) Typical N2 adsorption-desorption isotherms of a MOF (here UiO-66). (right) The CO2/CH4 gas separation ability of the same MOF, as indicated from the single
component gas adsorption isotherms.
(C) Applications of metal-organic frameworks in heterogeneous catalysis:
There is an increasing interest in employing MOFs as heterogeneous catalysts in oxidation, acid and base catalyzed reactions. Compared to homogeneous transition metal
catalysts, the heterogeneous MOF catalysts would simplify the work-up procedure by allowing simple filtration, facilitating product separation and catalyst regeneration.
MOFs have been used in heterogeneous catalysis employing several strategies. Probably the most studied and widely explored strategy is the utilization of the metal atoms
(saturated or unsaturated) of MOFs , which act as the catalytically active sites. Both single- and mixed-metal MOFs have been prepared by using mixture of metal salts during
the synthesis or by employing a post-synthetic metal-exchange strategy. A second methodology is based on the use of the metallated or metal-free organic ligands as active
sites. The third approach, derived from the large pore volume available in MOFs, consists of the incorporation of guest species (homogeneous metal-complexes and polyoxo-
metalates, metal nanoparticles, etc.) which serve as the active sites. Due to their lower physiochemical stabilities compared to zeolites, MOFs can not be employed in gas-
phase reactions. MOFs are suitable heterogeneous catalysts for the synthesis of fine chemicals requiring mild synthesis conditions.
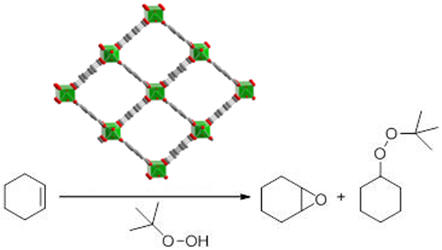
Figure 3. Representative example of a heterogeneous catalysis (epoxidation of cyclohexene ) involving a MOF catalyst (here MIL-47). A typical time-conversion plot of the
product (cyclohexene epoxide) is shown on the right.
References: (i) Chem. Soc. Rev., 2009, 38, 1201-1508. (ii) Chem. Rev., 2012, 112, 673-1268. (iii) Hu et al., Chem. Soc. Rev., 2014, 43, 5815. (iv) Müller-Buschbaum et al.,
Microporous Mesoporous Mater., 2015, 216, 171.
(v)
Li et al.,
Chem. Soc. Rev., 2009