Research Highlights
We have been involved in the synthesis of nitrogen heterocycles using picolylamine(s) and/or 2-cyanopyridine as the synthon(s). Some of the achievements are listed in the pages below:
- Reaction Schemes
- Structural Studies
- Scheme 7
- Scheme 6
- Scheme 5
- Scheme 4
- Scheme 3
- Scheme 2
- Scheme 1
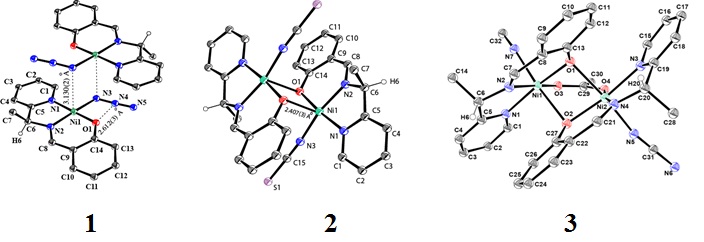
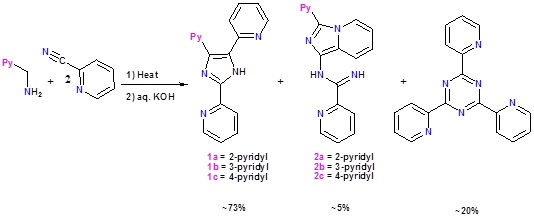
The 3-substituted imidazo[1,5-a]pyridine compounds having 1-(N-picolinamidin-2-yl) group (1a-1h) were synthesized by heating one equivalent of an aldehyde (8 examples), two equivalents of 2-cyanopyridine and ammonium acetate in PEG-400. By heating two equivalents of the aldehyde and one equivalent of 2-cyanopyridine under the same experimental conditions, 2,4,5-trisubstituted imidazoles (2a-2c) were isolated.
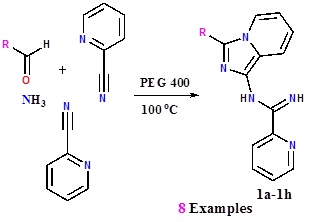
Heating 2-cyanopyridine and hydrazine hydrate at 100°C and reheating the resultant liquid with pyridine-2-carboxaldehyde yielded a red semi-solid. On adding aqueous KOH, a mixture of 1-(3,5-bis(2-pyridyl)-1,2,4-triazolyl)-3-(2-pyridyl)imidazo[1,5-a]pyridine (2a) and 1-((2-pyridyl)methanimine)-3-(2-pyridyl)imidazo[1,5-a]pyridine (2b) precipitated and from the filtrate 3,5-bis(2-pyridyl)-1,2,4-triazole (1) was isolated. Similar compounds were obtained from two other pyridinecarboxaldehydes.
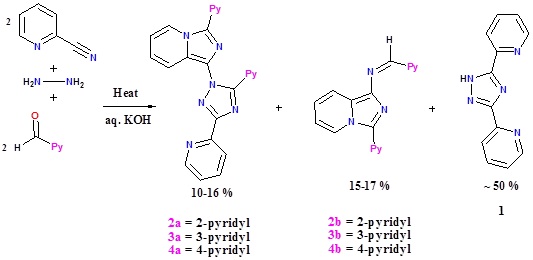
Heating a neat 1:2 mixture of 2-picolylamine and 2-cyanopyridine at 100°C, followed by treatment of the resultant red gummy substance with alkali resulted in the formation of 2,4,5-tris(2-pyridyl)imidazole (L3) as the major product and N-(3-(2-pyridyl)imidazo[1,5-a]pyridine)picolinamidine (L4) as the minor product.
By using 3-picolylamine and 4-picolylamine under the same experimental conditions, the respective imidazoles and imidazo[1,5-a]pyridines can be obtained. In all these reactions the triazine is also formed as a product.
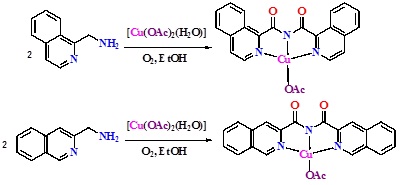
Ethanolic solution of ortho aminomethyl substituted isoquinolines, on stirring in air with half equivalent of [Cu(OAc)2(H2O)], respectively afforded [Cu(1-L)(OAc)] (1) and [Cu(3-L)(OAc)] (2) {1-L = bis(1-isoquinolylcarbonyl)amide ion and 3-L = bis(3-isoquinolylcarbonyl)amide ion}. Free ligands were isolated from the complexes shown in Schemes IV, III, II and I.
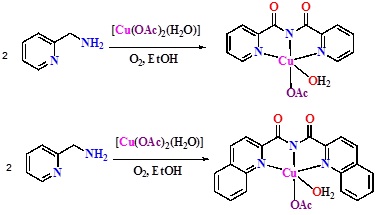
2-Picolylamine, in air and in hydrated ethanol (95%) on stirring with half equivalent of [Cu(OAc)2(H2O)] generated a green solution which on standing deposited blue crystals of composition [Cu(L2)(OAc)(H2O)].H2O in good yields. Under the same conditions by using 2-quinolylamine, green crystals of [Cu(L2')(OAc)(H2O)] was obtained, where in L2' is bis(2-quinolylcarbonyl)diimide ion.
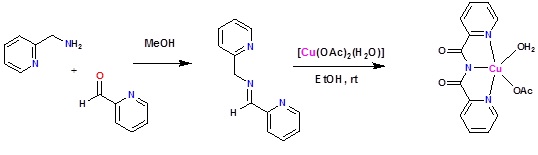
The Schiff base formed by the condensation of 2-picolylamine and pyridine-2-carboxaldehyde, when treated in ethanol with [Cu(OAc)2(H2O)]2 in air readily afford a blue colored crystalline solid, which has been structurally characterized as [Cu(L2)(OAc)(H2O)].H2O, where in L2 is bis(2-pyridylcarbonyl)diimide ion.
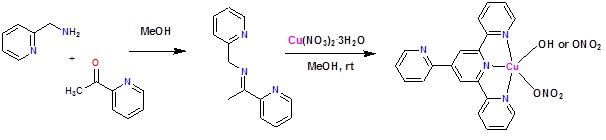
The Schiff base formed by the condensation of 2-picolylamine and 2-acetylpyridine, when treated in methanol with Cu(NO3)2.3H2O in air readily afford a green colored crystalline solid, which has been structurally characterized as {[Cu(L1)(OH)(NO3)][Cu(L1)(NO3)2]}.2H2O, where in L1 is 4'-(2-pyridyl)-2,2':6',2"-terpyridine.
- Structural Study 6
- Structural Study 5
- Structural Study 4
- Structural Study 3
- Structural Study 2
- Structural Study 1
A triad of bis-chelates of 4'-(2-pyridyl)-2,2':6',2"-terpyridine (L) having the formula, [M(L)2]Cl2.xH2O, {M = Ni, Fe, Ru and x = 8, 9, 10 respectively} were synthesized. All the three complexes crystallized in P space group and each contain the respective iso-structural [M(L)2]2+ species, two Cl– ions as well as the water molecules of crystallization. The water-chloride 2D-network are amalgamated between the hydrophobic layers of [M(L)2]2+ ions. The centro-symmetric repeating unit that makes the water-chloride 2D-network is of L5(2)5(3)8(2)4 pattern. Thermogravimetric profiles for the loss of water molecules are reported.
Schiff bases L1-L5 {N-[1-pyridine-2-ylethylidene]pyridine-2-amine (L1), 3-methyl-N-[1-pyridine-2-ylmethylidene]pyridine-2-amine (L2), 3-methyl-N-[1-pyridine-2-ylethylidene]pyridine-2-amine (L3), 4-methyl-N-[1-pyridine-2-ylmethylidene]pyridine-2-amine (L4), 4-methyl-N-[1-pyridine-2-ylethylidene]pyridine-2-amine (L5)} were synthesized and on reaction with Co(NO3)2.6H2O, complexes having the molecular formulae [Co(L1O)2]NO3 (1), [Co(L2O)2]NO3.xH2O (2a, x = 2; 2b, x = 3), [Co(L3O)2]NO3 (3), [Co(L4O)2]NO3.4H2O (4), [Co(L5O)2]NO3 (5) were isolated from the respective imines. The salt [Co(L2O)2]PF6 (2c) was obtained by treating 2 with KPF6. Complexes 1-5 were formed as a result of addition of one molecule of water across the imine function and the resultant alcohol binds in its deprotonated form. The alcoholate ion remained bound in a facial tridentate fashion to the low-spin cobalt(III). X-ray crystal structure determination confirmed the presence of trans-trans-trans-NANPO (A = aminopyridyl and P = pyridyl) disposition in 2a and cis-cis-trans-NANPO in 2b, 2c and 4. Water dimers in 2a, 2b, 4 and water-nitrate ion network in 2a were other notable features.
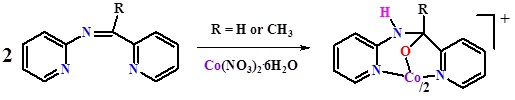
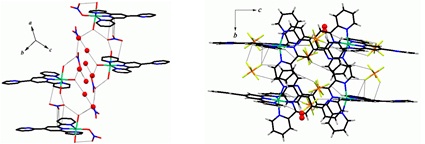
The mono- and bis- Ni(II) chelates of 4'-(2-pyridyl)-2,2':6',2"-terpyridine (L) having the molecular formula [[Ni(L)(H2O)3](NO3)2]3[Ni(L)(NO3)(H2O)2](NO3).5H2O (1) and [Ni(L)2](PF6)2.2H2O (2) respectively are synthesized. The structural, optical and magnetic characteristics are determined. The asymmetric unit of (1) contains three [Ni(L)(H2O)3]2+ (1a) and one [Ni(L)(NO3)(H2O)2]+ (1b) species along with five water molecules and seven nitrate ions. In both 1a and 1b, the bivalent nickel has pseudo-octahedral mer-N3O3 and in 2, mer-N3N3 environments. The molecular packing reveals the presence of water-nitrate ion network, (NO3-)---π interaction in 1 and F---π interaction in 2 and water dimer in both.
Three complexes of composition [CrLX3], where L = 4'-(2-Pyridyl)-2,2':6',2"-terpyridine and X = Cl-, N3-, NCS- are synthesized. They are characterized by IR, UV-Vis, fluorescence, EPR spectroscopic and X-ray crystallographic studies. Structural studies reveal that the Cr(III) ion is coordinated by three N atoms of L in a meridional fashion. The three anions occupy the other three coordination sites completing the mer-N3Cl3 (1) and mer-N3N3 (2 and 3), distorted octahedral geometry. The Cr-N2 has a shorter length than the Cr-N1 and Cr-N3 distances and the order Cr-N(NCS-) < Cr-N(N3-) < Cr-Cl is observed. They exhibit some of the d-d transitions in the visible and intra-ligand transitions in the UV regions. The lowest energy d-d transition follows the trend [CrLCl3] < [CrL(N3)3] < [CrL(NCS)3] consistent with the spectrochemical series. In DMF, they exhibit fluorescence having π → π* character. All the complexes show a rhombic splitting as well as zero-field splitting (zfs) in X-band EPR spectra at 77K
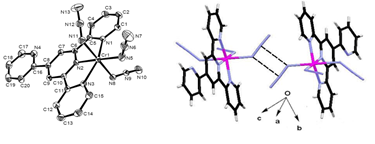
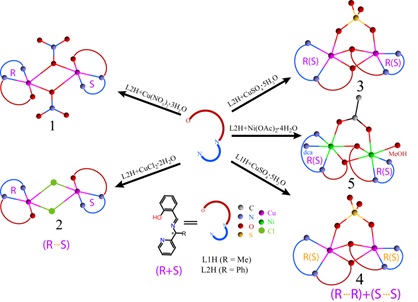
Using a racemic mixture of two chiral tridentate ligands 2-((1-(2-pyridyl)ethylimino)methyl)phenolate ion (L1) and 2-((phenyl(2-pyridyl)methylimino)methyl)phenolate ion (L2) and Cu(II) or Ni(II) salts, complexes having formulae [Cu(L2)((µ-1,1-NO3)]2 (1), [Cu(L2)((µ-Cl)]2 (2), [Cu2(L2)2(µ-1,2-SO4)] (3), [Cu2(L1)2(µ-1,2-SO4)] (4) and [Ni2(L2)2((-OAc)(N(CN)2)(MeOH)] (5) were synthesized and characterized. Determination of molecular structures of all the five complexes confirmed the presence of a dimetallic core constructed by monochelates of the ligands. Compound 1 and 2 have NO3- and Cl- bridge between two copper centers and 3-5 have phenoxo as well as SO42- (3,4) and OAc- (5) bridges. Compounds 1, 2 have center of inversion in the dicopper core and are heterochiral. Whereas, in 3-5 center of inversion do not lie in the dimetallic core and are homochiral. Compound 1 has a rare feature of µ-1,1-NO3- bridge between two copper centers.
Synthesis, structural characterization of complexes having the formula [Ni(L)(N3)], [Ni(L)(NCS)]2 and [Ni2(L)2(OAc)((NC)2N)].(CH3CN).(H2O) reveal the presence of (a) involvement of the electrophilic central nitrogen atom of coordinated azide in a OPδ----Nδ+ type dipolar interaction with the phenolate oxygen (b) existence of a distorted square-pyramidal coordination geometry around Ni(II) ion and (c) a co-ligand dependent enantiomeric or conglomeric dimerization of monochelates.