GREEN SOLVENT LABORATORY
@ Department of Chemical Engineering, IIT Guwahati, Guwahati, India.
Research
-
The Green Solvent Group uses experiments and molecular modelling insights into the use of Ionic Liquids (IL) and Deep Eutectic Solvents(DES)
as green solvents concerning both energy generation and environment mitigation. We adopt multiscale strategies which integrates molecular modelling
design to process simulation(ASPEN) for the development of industrial chemical products and processes. Some applications include applications such
as coal beneficiation, biomass dissolution, metal ion extraction, hydrogen energy generation and thermal energy storage. Recently we have started
evaluating DES as potential electrolytes and thermal fluids for supercapacitors and solar desalination respectively. We have also initiated
thermodynamic pathways using biocompatible gels comprising of Polysaccharide or DES based precursors for drug delivery.
We use both Quantum Chemical (Gaussian) and Molecular Dynamics (NAMD/AMBER) methods to predict thermodynamic and transport properties.
Other properties of interests are primarily in predicting activity coefficients for phase diagrams using Continuum Solvation Model such as
COSMO (COnductor like Screening MOdel). Other interests lie in the Reactive Force Field simulations (ReaxFF) of both renewable (alcohols) and
non-renewable (coal and chemical hydrides) energy sources. A brief description of my research areas which includes both Ionic Liquids and DES are
given below:
- Dearomatization of Light Naptha and Kerosene Fractions with DES.
Food Grade Quality Hexane is a light distillate produced from special cut Naptha where the hexane rich is extracted and then ensured required specification. It is primarily used for solvent extraction units for vegetable oil and spices. According to the specification the limit of the benzene in the food grade Hexane should be <250 mg/kg after aromatic extraction. Hence separation of Benzene and Hexane is one of the most challenging tasks in the chemical industry. Traditional distillation is infeasible for the separation, because of their close boiling points and azeotrope formation. Extractive distillation or azeotropic distillation are complex and consume a substantial amount of energy. Hence focus is on both Liquid Liquid extraction using both conventional and greener solvents.
Kerosene distillates from oil field (140-240 ºC) usually contain around 30-35% aromatics. Kerosene essentially rich in aromatics are extracted prior to its use as aviation fuel and illuminating kerosene. Low concentration of aromatics in kerosene is usually desired from straight run kerosene. Hence refining of kerosene fractions is performed to produce superior kerosene (SK) and aviation turbine fuel (ATF). These end products needs to meet the BIS specification which states the condition of smoke point, freezing point and aromatic content as the performance of the depends on these specifications. This is required as kerosene based detergents and jet aviation fuel requires aromatic content in ppm. One way is to open the ring of the aromatics for hydrogenation but then it uses severe operation conditions. Liquid Liquid Extraction is another method which selectively extract the aromatic moiety using solvents. Some of the conventional solvents include liquid sulphur dioxide, triethylene glycols, sulpholane, nitroalcohols and dimethyl sulphoxide. One such application concerning a Poly Aromatic Hydrocarbon extraction refers to the Scheme as below:
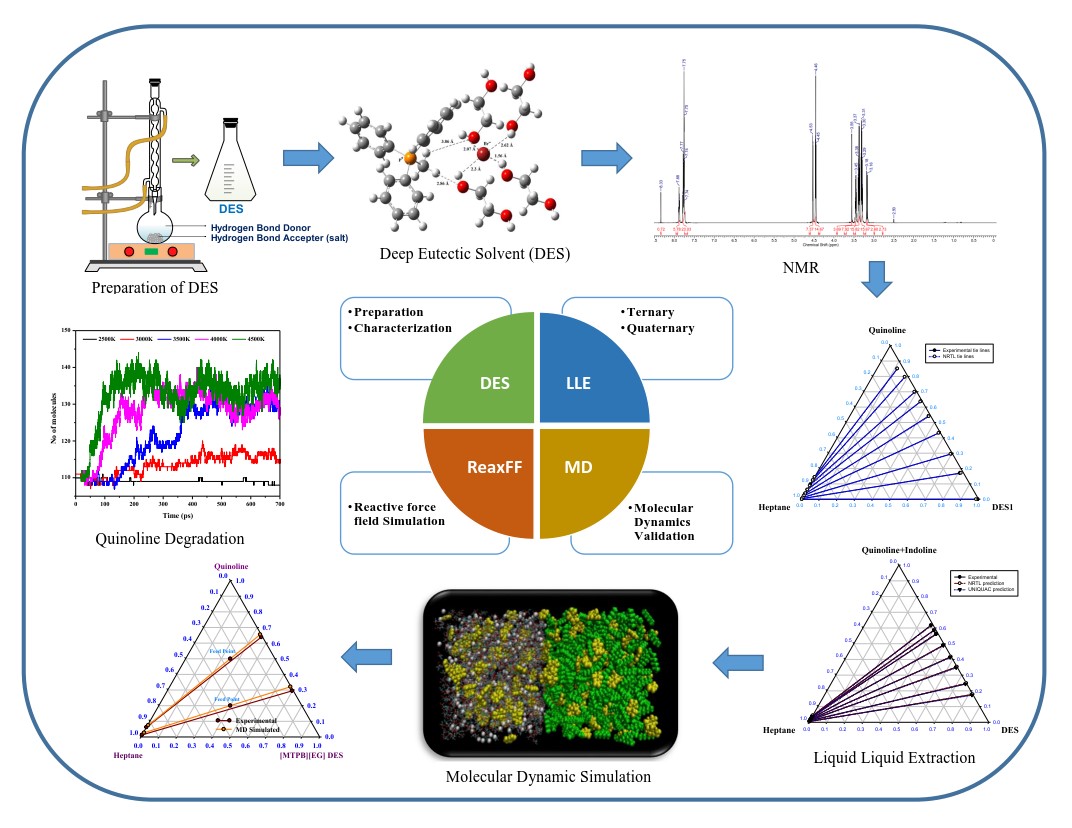
Figure 1: Extraction and Degradation Kinetics of Poly Aromatic Hydrocarbons (PAH).
- Development of Cellulose, Hemicellulose and Cyclodextrin and DES based Hydrogels.
Hydrogels retain water by hydration, osmotic or capillary forces which are counterbalanced by the forces exerted by the crosslinked structure to retain the network. The absorption of water depends on the internal transport and diffusion of network .The amount of entrapment depends on the external stimuli. Hydrogel resemble extracellular matrix due to the biocompatible nature of constituents, soft and rubbery structure and its high water retention capacity. For that, hydrogels are widely used in tissue engineering, cell encapsulation; and controlled delivery of drug and biomolecules. Apart from the biomedical applications, the functional moieties of hydrogel poses active binding sites for metal ions and dyes, leading to its application as biosorbent. The scheme below shows our recent progress in this area. We intend to use DES based hydrogels for water cleansing and extraction.
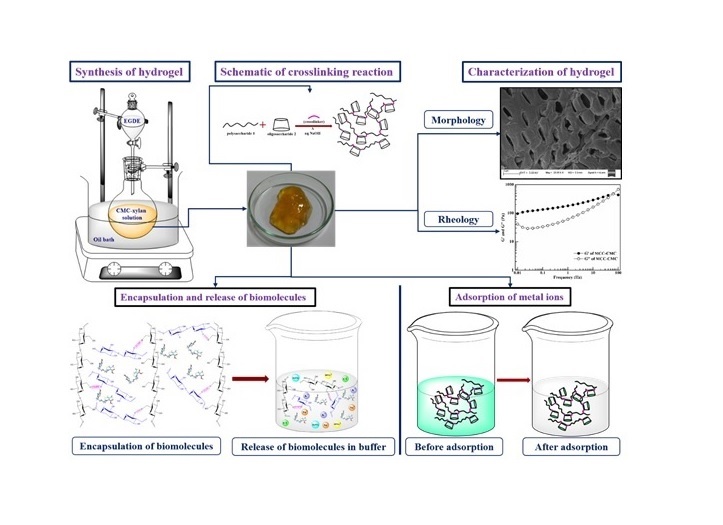
Figure 2: Development of Hydrogels.
- DES based Nanofluids for Concentrated Solar Power and Solar Desalination.
(a) Concentrated Solar Power (CSP) is one of the emerging renewable energy technologies, where sunlight is concentrated from a large area and stored in a collector filled with heat transfer fluid (HTF). In a CSP, increased heat transfer effect is a key deliverable which is usually obtained by enhancing the thermophysical properties of HTF. The HTF usually works as a heat storage and transfer media simultaneously. Some of the commercially used HTFs are Therminol VP1, synthetic oil and molten salt mixtures. However, these HTFs have some drawbacks like high vapor pressure, high melting point and their corrosive nature. With added Nanoparticles they become nanofluids and gives a higher heat transfer capacity. The scheme below shows the updated progress which includes actual forced convention experiments and ASPEN simulations.
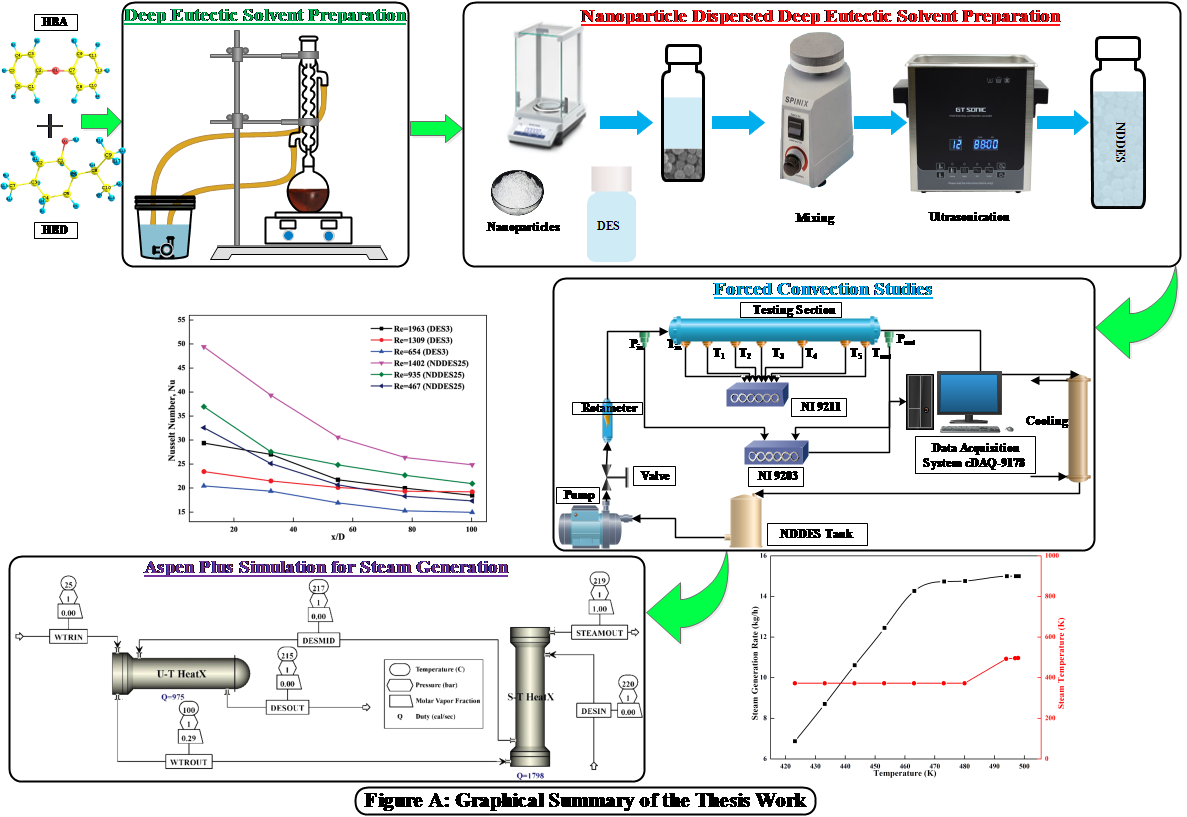
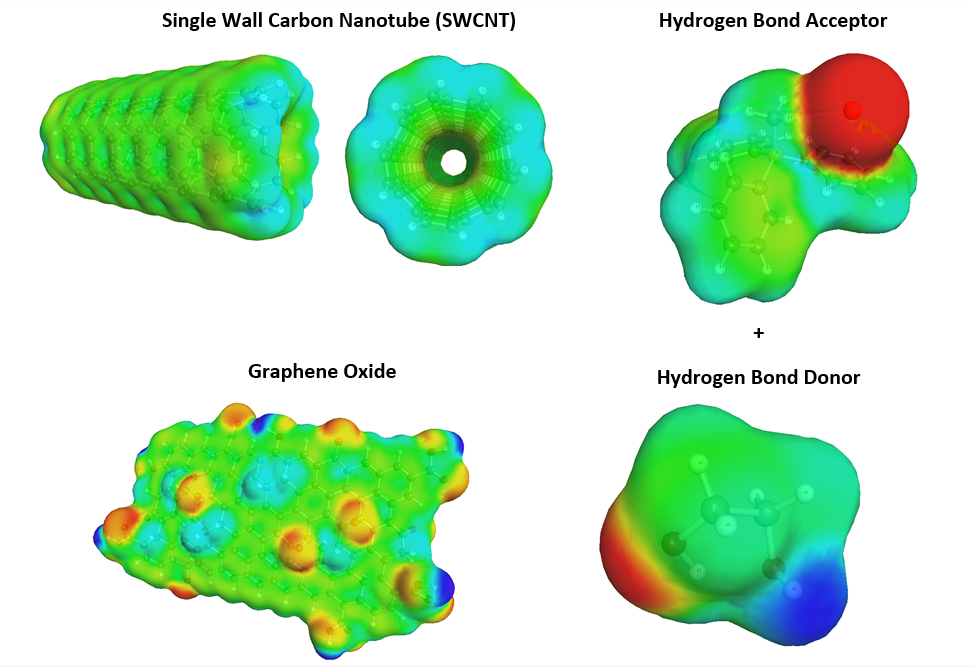
(b) The stability of Nanofluid based thermal energy storage systems without clogging is a prime concern for maintaining their thermal conductivity for a longer time span. We intend to conjugate different functionalized MWCNTs (Multi Walled Carbon Nanotube) with either HBA (Hydrogen Bond Acceptor) or HBD (Hydrogen Bond Donor) molecules due to their extended π-electron clouds. Presently such conjugation result in faster heat transition and better stability in the matrix due to their interactions with the DES medium. This DST sponsored project thus attempts to design DES as base fluid along with graphene coated carbon nanotube and functionalized MWCNTs as nanoparticles. Thereafter these nanofluids shall be evaluated in a multistage flash (MSF).In the case of Solar Desalination, the nanoparticles namely MWCNT,GO and the DES shall accessed prior to use in simulators such as ASPEN. Here they are inserted as Pseudocomponent using a combination of Quantum Chemical and ASPEN suite of programs. The COSMO Solvated Surfaces are created in B3LYP/6-31G* using the SCRF=COSMORS