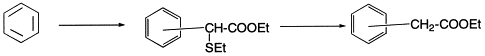Synthesis of ethyl arylacetates by means of Friedel–Crafts reactions of aromatic compounds with ethyl α-chloro-α-(ethylthio)acetate catalysed by ytterbium triflate
Abstract
An efficient Friedel–Crafts alkylation of aromatic compounds with ethyl α-chloro-α-(ethylthio)acetate catalysed by ytterbium triflate, followed by desulfurisation of the product provides a convenient methodology for the synthesis of ethyl arylacetates of aromatic and heteroaromatic compounds.
Keywords
- catalysis;
- Friedel–Crafts reaction;
- lanthanides;
- substitution
The Friedel–Crafts reaction is generally achieved using a Lewis acid as the catalyst. A common problem, particularly in industrial processes, is that the Friedel–Crafts reaction requires stoichiometric amounts of the Lewis acid which cannot be reused because of its instability in the usual aqueous work up.
Lanthanide trifluoromethanesulfonates1 [lanthanide triflates; Ln(OTf)3] work efficiently as versatile Lewis acids and have been employed in a number of reactions both in organic and aqueous media in catalytic quantities. Since the first utilisation of Yb(OTf)3 by Forsberg et al.,2 it has found wide utility in organic synthesis and recently Kobayashi et al.1a have used Yb(OTf)3 as the catalyst in Friedel–Crafts acylation reactions. It was demonstrated in these reactions that the catalysts were easily recovered after the reactions were completed and could be reused.
As one of many synthetic applications of α-chlorosulfides, Tamura3 has accomplished the preparation of arylacetates by the Friedel–Crafts reaction of ethyl α-chloro-α-(methylthio)acetate in the presence of stoichiometric quantities of a Lewis acid such as SnCl4 or TiCl4. Subsequently, this methodology has been extended by Arai4 for the synthesis of naproxen.
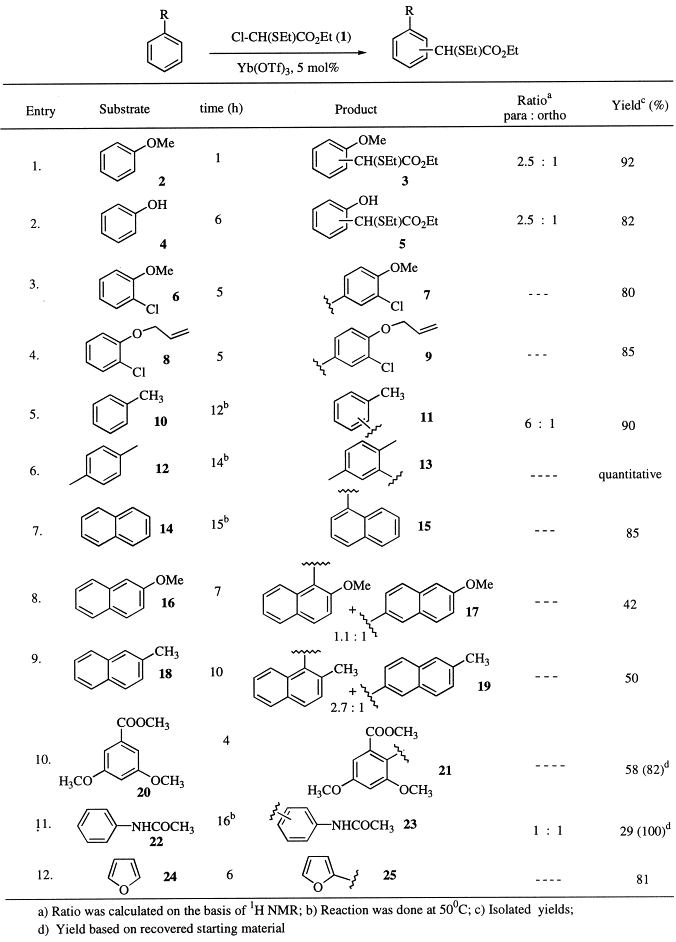
Herein, we describe an excellent preparative method for the synthesis of ethyl aryl-acetates by Friedel–Crafts reactions of aromatic compounds with ethyl α-chloro-α-(ethylthio)acetate 1 using Yb(OTf)3 as the right catalyst, followed by desulfurisation of the resulting ethyl-α-(ethylthio)arylacetates (Eq. (1)). The results in this regard are presented in Table 1.
- Table 1.
- Full-size table
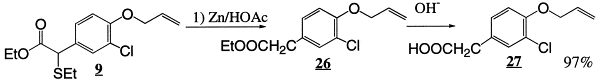
Initially, the reaction of anisole 2 with ethyl α-chloro-α-(ethylthio)acetate 1 (1.1 equiv.) in the presence of Yb(OTf)3 (5 mol%) was carried out in CH3NO2 at room temperature to give the product 3 in 92% yield (para:ortho 2.5:1). In the case of phenol 4, the reaction also proceeded smoothly at room temperature and afforded the product 5 in excellent yield. This method has also been applied to aryl alkyl ethers such as 6 and 8 and the products 7 and 9, respectively, were isolated in excellent yields. It is worth mentioning that the allyl group in 9 survived the reaction conditions. Compound 9 after desulfurisation and saponification afforded the anti-inflammatory agent, alclofenac 27 3 in 97% yield.
This reaction has been extended to substituted benzenes such as toluene 10 and xylene 12 (50°, 12–14 h) and the corresponding products 11 and 13 were obtained in almost quantitative yields. Next, we examined the reaction of naphthalene, 14 with 1 and isolated the α-substituted product 15 in 85% yield. In the case of substituted naphthalenes such as 16 and 18, the corresponding products 17 and 19, respectively, were obtained in moderate yields as a mixture of 1 and 6-substituted products. The reactivity of substrate 22 containing the acetamido group was much slower under the reaction conditions and gave the product 23 with poor conversion. The reaction of methyl 3,5-dimethoxybenzoate 20 with 1 was very sluggish at room temperature but at 50°C the ortho product 21 was obtained in good yield, the methyl ester surviving the reaction conditions.